Distilling 101
A Complete Guide To Distillation Temperatures (Explained!)
If you’re anything like me, the further you get into the distilling hobby, the more you whish you had a degree in chemical engineering..
When people start talking ‘Azeotropes’, ‘temperature gradients’, and ‘equilibirum points’.. its clear that temperature plays a crucial role in the distilling process – but what does this all mean!?
In this article, we’re going to discuss the key temperature points when distilling alcohol, how to measure them, and what these temperatures mean for your moonshine.
Table of Contents
What temperature do different alcohols boil at?
First, we need to understand the chemistry of alcohol.
Ethanol is a monohydric primary alcohol. This allows the volatile substance to melt at negative 117.3 degrees Celsius and boils at 78.5 degrees Celsius. Ethanol mixes without separation, with water in all proportions and this then is separated from water without interruption. Hence, ethanol that is completely free of water in all proportions is called absolute ethanol.
A general fact is noted that all alcohol products evaporate but at different speed levels. For instance, ethanol evaporates about five times faster than water. Beer can lose 30% of its alcohol in 12 hours, while wine can lose 1% of its alcohol boiling at the temperature of 78.15 degrees Celsius -78.37 degrees Celsius (173 F), and 70% of ethanol can evaporate in 30 seconds.
| Product | Boiling point |
|---|---|
| Acetone | 56.5C (134F) |
| Methanol (Wood Alcohol) | 64 C (147F) |
| Ethyl acetate | 77.1C (171F) |
| Ethanol Azeotrope | 78.2C (172.8F) |
| Ethanol | 78.4C (173.1F) |
| 2-Propanol (rubbing alcohol) | 82C (180F) |
| 1-Propanol | 97C (207F) |
| Water | 100C (212F) |
| Butanol | 116C (241F) |
| Furfural | 161C (322F) |
What Is An Azeotrope?
The word azeotrope is synonymous with constant boiling mixtures. Therefore, according to the Oxford dictionary, azeotrope/azeotropic is derived from chemistry terminology, which agrees that it means a mixture of liquids in which the boiling point remains the same throughout the distillation process, at a given pressure without change in composition.
An understanding of azeotrope is interesting as they often occur in separate fractional distillation and make a given separation impossible by ordinary rectification. Secondly, they may be utilized to separate mixtures, not ordinarily separable by simple straight rectification.

What is the azeotropic temperature of ethanol?
The azeotropic temperature of ethanol is the temperature at which an azeotropic mixture of ethanol and water is formed
The azeotropic temperature of ethanol depends on the concentration of ethanol in the mixture. For example, the azeotropic temperature of a mixture of 95% ethanol and 5% water is 78.15°C. At this temperature, the mixture boils at a constant temperature and the vapor produced by the boiling mixture is composed of 95% ethanol and 5% water, regardless of the composition of the liquid mixture.
Here is a table of the azeotropic temperature of ethanol for each 10% ABV increment:
| Ethanol Concentration | Azeotropic Temperature |
|---|---|
| 10% | 78.5°C |
| 20% | 78.3°C |
| 30% | 78.2°C |
| 40% | 78.1°C |
| 50% | 78.0°C |
| 60% | 77.9°C |
| 70% | 77.8°C |
| 80% | 77.6°C |
| 90% | 77.5°C |
| 100% | N/A |
Note that the azeotropic temperature of ethanol decreases as the ethanol concentration increases. Also, the azeotropic temperature is not defined for pure ethanol, because pure ethanol does not form an azeotropic mixture with water..
How Does The Distilling Temperature Of Ethanol Change With Concentration?
This is probably one of the most helpful things I found when learning to distill.
The temperature of your mixture will change based on the alcohol percentage by volume.
Example:
If you charge your still with 40% ABV low wines, it will boil at 84C. however, If you charge you still with a 10% ABV sugar wash, it wont boil until it reaches 93C.
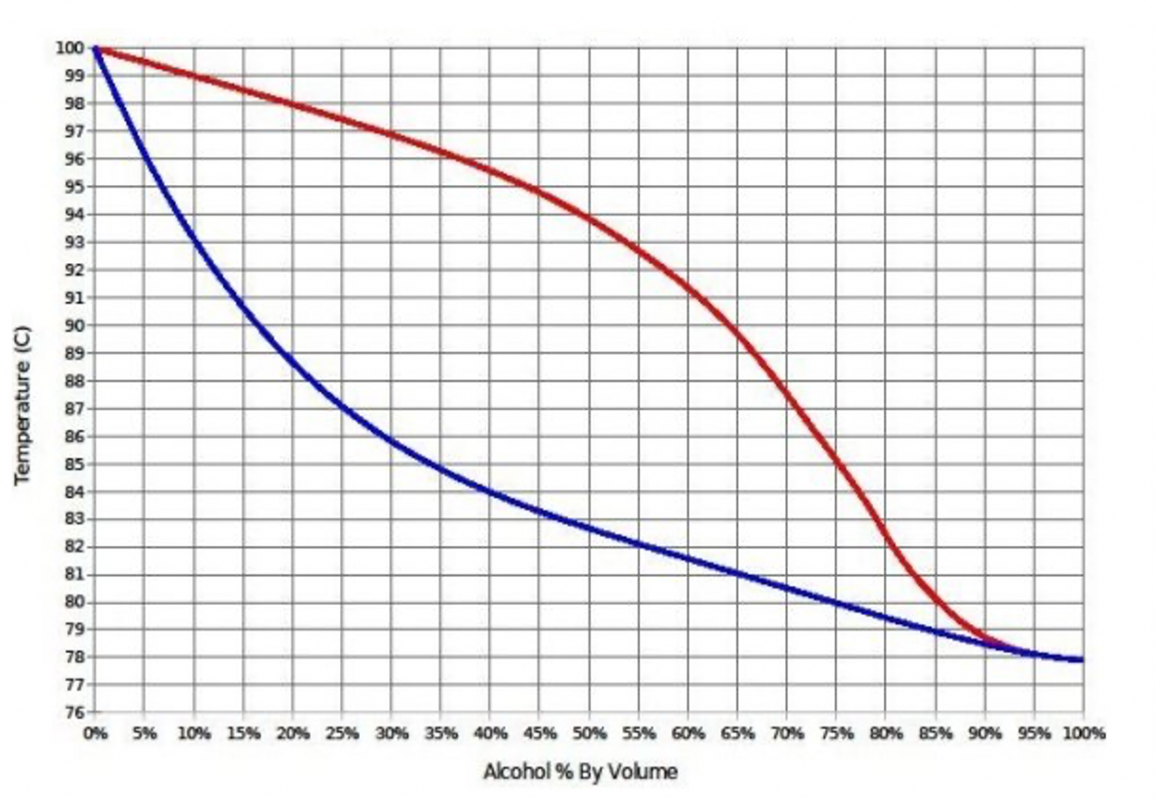
The key idea here is you can actually tell how far through fermentation you are from the temperature of the boiler!
Where should we be measuring the temperature?
What is the difference between vapor temperature and solution temperature?
The solution temperature is measured inside the boiler. Whereas, the vapor temperature is measured in the head/ column or still just before the condenser.
Measuring Temperature Inside The Boiler:
In the boiler, we really need to know the temperature of the liquid low wines or wash. Thant means placing the probe submerged rather than in the headspace of the boiler.
The temperature of the boiler can tell us a few things:
- How fast the still is heating up, and when we approaching distillation temperature: This is useful as the column temperature won’t change until you start boiling (~ 78 degrees).
- How far through the distillation process you are: By measuring the temperature the wash/wines are boiling at we can infer the ABV that is remaining, and therefore know how far along the run we are. For example; if we start distilling with 40% ABV low wines the boiler temperature will begin at 84 degrees C. If we’re almost finished with the run and the temperature has raised to 93 degrees C, we can infer the boiler now contains 10% ABV.
Measiuring Temperature Inside The Column:
Measuring the temperature inside the column will tell us the vapor temperature.
Other Location To Measure Temperature:
I want to know exactly what’s happening during the distilling run, and to collect this data. OTher places to consider measuring temperature include:
- The incoming cooling water
- The outgoing cooling water
- The headspace of the boiler (to see when boiling starts)
- The product temperature (to make sure you’re fully condensing product)
How does the temperature change through a Pot Distillation Run?
When distilling alcohol, the various components of the distillate are often referred to as the “fores,” “heads,” “hearts,” and “tails.” These terms refer to the different stages of the distillation process, and the temperature at which each component is collected can vary depending on the specific distillation method being used and the desired outcome of the distillation.
The “fores” are the first portion of the distillate to be collected. They typically have a higher concentration of impurities and are considered to be of lower quality than the other components of the distillate. The “heads” are the next portion of the distillate to be collected, and they are typically richer in aroma and flavor than the “fores.” The “hearts” are the central portion of the distillate, and they are typically the highest quality and most desirable part of the distillate. The “tails” are the final portion of the distillate to be collected, and they typically have a lower concentration of ethanol and a higher concentration of impurities than the other components of the distillate.
The temperature at which each of these components is collected can vary depending on the specific distillation method being used and the desired outcome of the distillation. In general, the “fores” and “heads” are collected at lower temperatures, while the “hearts” and “tails” are collected at higher temperatures. The temperature at which each component is collected can be determined by monitoring the temperature of the vapor being produced by the pot and adjusting the flow of vapor through the condenser as needed to collect the desired portion of the distillate.
| Distillation Stage | Aprrox Temperature |
|---|---|
| Fores | |
| Heads | |
| Equalisation | |
| Hearts | |
| Tails |
Fores
Heads
It is essential to know when exactly to start and when to end collecting moonshine from your still. An indication of alertness is when you start to see some products dripping from your still when the head/column temperature reaches 56 degrees Celsius.
Generally, between 78-82 degrees Celsius is the temperature range in which one is safe to collect the moonshine at a head temperature of 94 degrees Celsius or higher.
Hearts
Tails
How does the distillation temperature change through a Reflux Distillation run?
| Distillation Stage | Aprrox Temperature |
|---|---|
| Fores | |
| Heads | |
| Equalisation | |
| Hearts | |
| Tails |
Fores
Heads
Equalization
Hearts
Tails
Coming Back To The Question, What is the best temperature for distillation?
Well..
As noted in the table above, the pure ethanol boiling temperature is 172 degrees Fahrenheit. When distilling, changes are yet to occur because the ethanol in the wash is diluted by other products, mainly water. This directly affects the boiling temperature, because the more the water in the solution, the higher the boiling point of the mixture will become.
This can be indicated by inserting a thermometer into your boiler and measuring the distillation temperature as it boils. Note the temperature when your still start producing ethanol will be higher than the boiling point of ethanol.
Distilling Temperatures: Frequently Asked Questions
Below are some of the questions we’ve had asked in relation to distilling temperatures
Q. What temperature should a still run at?
This depends on the type of still, and what you’re making. A reflux still that is producing good ethanol and is properly equalized should run close to 78.2C. A pot still making rum, gin or whiskey will typically start the distillation run at around 80C and slowly move up to 95C as the distillation run progresses.
Q. What temperature should moonshine be kept at?
We suggest any distillate (moonshine) is kept in a cool dark place away from large temperature swings and direct sunlight which can degrade some of the flavor compounds in the bottle.
In short, direct sunlight is bad, and in a dark pantry cupboard is good.
When aging spirits like whiskey, rum, and brandy in barrels these are usually kept in a warehouse between 10 and 18 degrees C. A natural seasonal warming and cooling of about 5 degrees cause the barrels to expand and contract to help transfer flavor from the wood into the spirit.
Vodka and gin can be kept in the freezer as these drinks are often best enjoyed cold or on ice.
Q. What temperature should distillate be collected at?
Ideally, your distillate should come off the still at as close to room temperature as possible.
Hot product is a sign that your condenser is undersized, or your cooling water is too hot or too slow.
Hot product will continue to evaporate, meaning that you loose your precious hooch to the atmosphere, and it also makes taking accurate alcoholmeter readings really difficult.
Q. What happens if my distillation temperature is too high?
As we’ve (hopefully) managed to explain in this article, you cant force the distillation temperature to be high or low – it is what it is based off the chemistry inside your still at the time.
If your distillation temp is too high, it will mean you’re further through the distillation process than you may be expecting. That is; your boiler ABV has dropped, or, you’re moving into the tail end of the run.
Q. How To Correct For Temperature When Taking Alcoholmeter Readings.
If you have purchased an alcoholometer from your local brew shop or online, they are accompanied by a correction table.
You have finalized your reflux still and collected the spirit using an alcoholometer. You observed readings of 93% abv and you monitored the temperature of the spirit at 30%. Following the steps and the table below, let’s calculate the accurate % abv of your spirit:
Image shows proof hydrometer temperature correction table

- Step 1: At the top of the table in the first column t reads numbers 5-30. This represents the temperature of the spirit. So we noted the current temperature at 30 degrees Celsius. Go to the 30th number at the end of the row.
- Step 2: Now the hydrometer measured 93% alcohol. Using your finger, move horizontally till you come across 93.3( this is the closest reading to the measurement).
- Step 3: Now follow the row over to the left where the 93.3 number is listed until you get to the 20 degrees Celsius row (highlighted in gray to make it easier). You will read 91. This means there is actual proof of 91% abv.
To verify your experiment, let your moonshine cool to 20 degrees Celsius and see how accurate your distillation temperature measure was. You can also place your mixture in a cool place such as the freezer and retake the measurement of your product using a thermometer. You will note how temperature can affect the reading of the distillation temperature.
Estimated Alcohol hydrometer temperature correction
A simpler and fairly accurate method to estimate the alcohol percentage in your mixture is to remember one general rule. For every 1 degree Celsius over 20 degrees Celsius, subtract 0.33 from your alcohol meter reading. For example, the actual temperature = 30 degrees Celsius, the observed alcohol meter temperature = 93% , therefore 93% -(0.33* 10 degrees Celsius = 90.7 abv).
Sources
- https://www.infoplease.com/encyclopedia/science/chemistry/organic/ethanol
- https://foodwine.com/alcohol-evaporation/R.H Ewell, J.M Harrison and Lloyd Berg (October 1944).Azeotripic Distillation pg 871-874. Vol.36 No.10
Related Posts
Why Is My Moonshine Cloudy? (How to Fix Cloudy Distillate)
So, you’re finally done with your spirit run and can’t wait to get your hands{...}
How To Make Watermelon Moonshine (The Right Way!)
Watermelon moonshine is a fruity, delicious, and sweet drink you can make at home with{...}
The Best Oak For Aging Whiskey
Oaking or ‘aging’ whiskey is what gives it all of it’s color, and a lot{...}
Why is Moonshine Dangerous (Must Read)
Moonshine is synonymous with illegal and highly potent hooch that’s been distilled in the dead{...}












Pingback: Homepage
Pingback: สล็อตวอเลท
Pingback: travestis málaga
Pingback: adhd virginia
Pingback: recuperatoare caldura
Pingback: Devops Services company
Pingback: Burma magic mushrooms
Pingback: ai ดูดวง
Pingback: คาเฟ่ ชลบุรี
Pingback: plumber woodstock ga
Pingback: 123movies