Distilling 101, Safety
How to Avoid Methanol When Distilling Alcohol (Must Read!)
Making your own spirits at home is not only interesting but also a great learning experience. However, preparing any alcoholic beverage by yourself calls for the right care and precision.
Methanol is an unwanted byproduct of fermentation.
It can be concentrated if distillation is performed incorrectly and this can lead to harmful or even lethal concentrations in your final product.
Fortunately, you can reduce the amount of methanol produced by avoiding fermenting fruits that are high in pectin and doing so with healthy yeast at controlled temperatures. Then, when distilling, make careful cuts to remove the ‘fores’ and ‘heads’ which contain the methanol at the start of distillation.
Read on to learn how!
Table of Contents
How is Methanol Produced?
Also known as methyl alcohol or wood spirit, methanol is the simplest (shortest chain) of alcohols which is one-part carbon, one-part oxygen and four parts hydrogen.
Methanol is commonly produced commercially from coal, natural gas, and other renewable sources such as recycled carbon dioxide, biomass, and municipal waste. Initially, it was produced by performing a destructive distillation of wood, but nowadays it’s produced from synthesis gas by combining hydrogen and carbon monoxide with the help of a catalyst.
Apart from that, methanol is also produced in small amounts during the process of alcohol fermentation. It’s actually a misnomer that distilling ‘produces’ methanol. Distilling can only concentrate the methanol that is already present if done incorrectly, or remove the methanol from the product when done right.
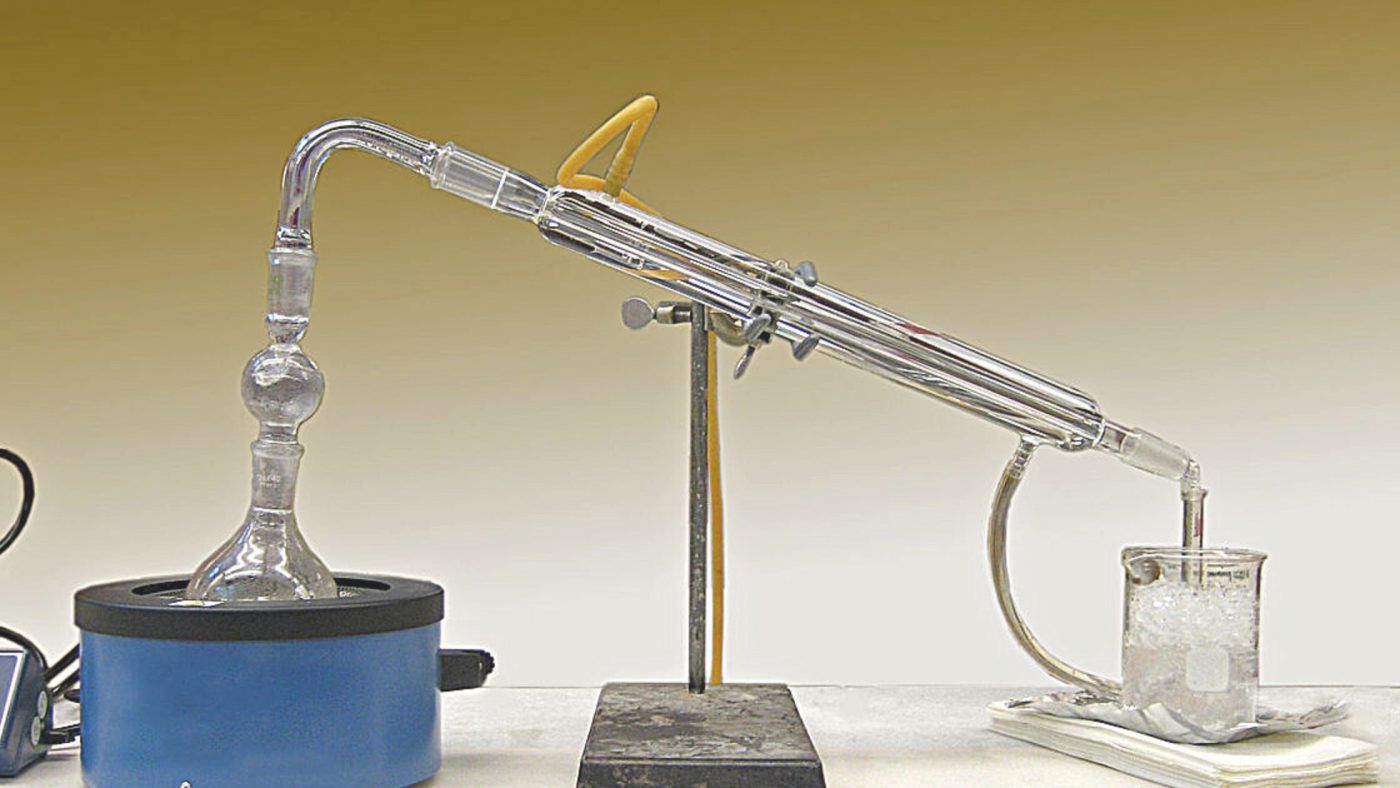
How to Minimize Methanol in Fermentation?
When making alcohol at home, it’s the fermentation stage that produces methanol.
The amount produced will vary with different conditions, including the temperature, the type of yeast and other bacterial in the solution, the type of food you provide to them, the minerals, and more.
Normal fermentation of starch-derived sugars from corn, wheat, and barley will only contain very small amounts of compounds that will turn into methanol during fermentation.
What you need to watch out for is fermenting foods containing lots of pectin as this is the leading cause of methanol in the final product.
Pectin is commonly found in fruits – particularly in the skins – and in lesser ripe fruit as it gets converted during the ripening phase. Fruits like cherries and grapes have quite low pectin, while circuit peels like on grapefruit and lemons contain very high pectin.
| Fruit | Typical Pectin Content |
| Cherries | 0.4% |
| Grapes | 0.2% – 0.6% |
| Oranges | 0.5–3.5% |
| Apricots | 1% |
| Apples | 1–1.5% |
| Grapefruit | 3.9% |
| Rose hips | 15% |
| Citrus peels | 30% |
A couple of general rules to minimize methanol when distilling are:
- Be warier of methanol when distilling fruits, as opposed to sugar washes, grain washes, or whiskeys.
- Brandy made from distilled fermented grapes will have a lower methanol content than schnapps (fruit brandy) made from fermented apples, or citrus.
- Remove pectin from the food sources you are using, or simply go for items that don’t contain pectin. This prevents microorganisms from producing methanol.
- Use high-quality, reliable yeast. Turbo can create more methanol and unwanted flavors so decide if turbo yeasts are a good option.
You can find out more on yeast below: - Make sure to control the temperature and don’t let it go too high during fermentation. Check out this guide on the best temperature controllers for fermentation here.
- Sterilize everything before fermentation to kill harmful bacteria.
Can you Test for Methanol in Alcohol?
Yes! You can test for the presence of methanol in an alcoholic beverage by performing some quick tests.
The Smell Test
Smelling the beverage is the easiest methanol test yet one that takes a lot of practice to hone your senses. If you sense an unpleasant chemical odor from the solution, the drink is not safe for consumption.
Methanol has a sharp, stinging scent that is quite potent and can be easily recognized as ‘the smell of alcohol’
Ethanol will smell much milder by contrast. It’s softer, less stingy, and almost has a ‘creamy’ aroma. Ethanol won’t smell as potent as methanol of the same concentration.
Compared to methanol, ethanol will smell very pleasant – but it’s very hard to tell if you’re not comparing the two side by side.
The Flame Test
Take a small sample of the alcohol solution and light it on fire – if you witness a yellow fire instead of a blue flame, the solution contains methanol.
Again, this test is good in theory, but in practice you’ll seldom be comparing two solutions that are entirely methanol or ethanol. They will be blended and of varying proportions.
Also, be very careful setting things on fire as distilling is a potentially explosive process. Keep any open flame well away from a running still.
The Chemical Test
A more effective test for methanol in alcohol is to apply sodium dichromate to a small sample of the solution.
All you need to do is mix 8 mL of a sodium dichromate with 4 mL of sulfuric acid, further swirling the mix and adding 10 drops of the same to a small container or a test tube containing the alcohol to be tested.
Gently swirl test tube, followed by using your hand to fan the air from the opening of the test tube towards your nose while you hold the tube 10-12 inches from your nose. Notice the smell – if it’s unpleasant and pungent, then the alcohol contains methanol. However, if it seems fruity, the beverage contains only ethanol and is safe for consumption.
How to Avoid Methanol in Distilling by Making ‘Cuts’?
This is where the magic happens.
Depending on the varying boiling points of different chemicals involved in distillation, the process allows you to collect those chemicals separately.
Lighter compounds with lower boiling points will boil off first. Heavier compounds with higher boiling points evaporate last.
Regular distillation (or pot distillation) allows this to happen very crudely.
Fractional distillation using a reflux still lets you separate the compounds much more accurately.
Making ‘Cuts’ is the process of collecting the take-off from your still in discrete containers. Changing between the containers at regular intervals (say every 200ml) while collecting the distillate in order to separate the output into four stages – foreshots, heads, hearts and tails.
At the time of distilling, you must collect into many separate glass containers as the flavor and contents of the alcohol solution undergo changes throughout the entire distilling.
Before the vapor temperature hits 175 degrees Fahrenheit the first to come out of the distill are the foreshots and this is the harmful stuff that contains mainly acetone (ethyl acetate), methanol and several other poisonous elements.
This part is highly toxic and tastes awful, which is why it must be separated out as methanol can lead to blindness and even death. The foreshots contain almost zero ethanol and must always be discarded.
It’s recommended to collect and discard around 4 ounces of foreshots per 5 gallons that are being distilled. However, this is the minimum recommendation, and you can always discard a little extra. If using a reflux still, it’s best to discard the initial 50 mL you collect, while throwing away 100-200 mL when using a pot still. This ensures that you get rid of all the methanol and other harmful foreshots.
The next stage is the heads, which come out at 175-185 degrees Fahrenheit. The heads are set aside but can be recycled, as they can contain substances that can affect the flavor of the final beverage. Heads will have a small amount of methanol, mixed with ethanol as the delineation is very gradual.
The hearts evaporate at 173 degrees Fahrenheit (78.3 degrees Celsius) and mainly contain ethanol. The majority of your take off will be heads.
If not cut on time, the unwanted bitterness and oily aroma from the tails could dominate. The tails come off at approximately 203°F (95°C) and should be removed from the distillate.
These can be collected and recycled to get a bit more ethanol in your next run.
How Much Methanol Can Someone Consume Before it’s Dangerous?
A dosage of as little as 10-30 mL of pure methanol can lead to methanol poisoning and be lethal.
Methanol is metabolized into formic acid when consumed, which can destroy the optic nerve or lead to permanent blindness.
The exact amount can vary from one person to another based on many factors such as their size, body fat, and how much they’ve drunk and eaten.
No matter the quantity, it’s an industrial chemical and must not be consumed under any circumstances.
Read more about the symptoms of methanol poisoning and how to treat it.
How Much Methanol is Usually Produced by Home Distillers?
Typically, home distillers produce around 0.0067% of methanol in their wash.
In other words, you will get 2 or 3 milligrams per litre of methanol produced by a home distiller during fermentation.
However, if improper cuts are made this methanol can be greatly concentrated during distillation.
Fortunately, based on these figures, a fatal dose of methanol would only be produced by distilling around 150 liters of liquid, which is likely far out of the usual capacity of home-distillers.
That’s why, even if a home distiller is not aware about the need to throw away the initial small amount of liquid, the little dose of methanol produced during home distilling will unlikely be enough to prove hazardous.
How Much Methanol Is There In Commercial Spirits?
Contrary to popular belief, commercial spirits are not methanol free. In fact, they can contain often higher levels of methanol than a well-made home-distilled drink.
The table below shows some typical methanol concentrations per liter of fluid in some common alcoholic drinks. If you’re interested in learning more, check out our complete article on how much methanol there is in commercially produced drinks.
| Type of alcohol | Methanol Content Per Drink (Average) |
| Beer | 6-27 mg/l |
| Wine | 40-321 mg/l |
| Whiskey | 80–260 mg/l |
| Cider | 200-400 mg/l |
| Vodka | 100 mg/l |
| Brandy | Up to2800mg/l |
| Tequila | Up to 3000 mg/L |
| Gin | 50 mg/l |
Conclusion
Taking a look at these measures, it can be rightly concluded that with a handful of precautions during the fermentation process you can easily ensure that you’ll make a wash that is safe from \high levels of methanol.
Furthermore, a little patience and skill is all it takes to achieve the right separation of the cuts – allowing you to discard all the bad stuff, and keep all the good.
By doing this you’ll end up with a drink that not only is perfectly safe to drink but also tastes and smells better too.
However, there’s always scope for error, so our final take away is as long as you stick to small volumes, its unlikely home distillers can produce anywhere near the amount of methanol required to cause harm.
Have fun, keep learning, and happy distilling.









Good advice thanks
Hello to you and your team
I am very happy to have great education and guidance from you.
with best wishes
Abby
Awesome advice, thanks
Really great advice but my still only holds 3 gallon, so what I normally make is
5 gallon of sugar wash and divide it into two 2 and a half gallon contents in the still so how much foreshots and heads will I have to take out of my 2and a half still contents.
good article
one question I have is will pectolase have any effect on the amount of methyl alcohol produced? I have no qualms about taking off the foreshots as they are useful as a cleaning solution for electrical and electronic components but am curious
I would say if you’re fermenting fruit then yes, add Pectinase to reduce the resulting methanol. But for a regualr sugar wash i dont think it will make much of a difference. There are a number of compounds aside from methanol that make up your foreshots (like ethyl acetate) that also need to be removed and the best method is by making good clean cuts. Cheers!